| 1. 4 | 9. 2 | 17. 1 | 25. 4 |
| 2. 2 | 10. 4 | 18. 3 | 26. 3 |
| 3. 3 | 11. 2 | 19. 4 | 27. 3 |
| 4. 3 | 12. 4 | 20. 3 | 28. 4 |
| 5. 4 | 13. 3 | 21. 1 | 29. 3 |
| 6. 3 | 14. 3 | 22. 1 | 30. 1 |
| 7. 1 | 15. 2 | 23. 4 | 31. 4 |
| 8. 3 | 16. 2 | 24. 2 | 32. 4 |
33. Ca +2 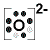 the O should have 8 dots
the O should have 8 dots
H-Br: the Br should have 6 dots
O=C=O each O should have 4 dots
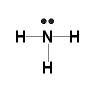
34. 
35. CCl4 is a nonpolar molecule because it has symmetrical distribution of charge
36. Ammonia is a polar molecule CCl4 is non polar, polar molecules have stronger imf's than non polar molecules
37. KCl has ionic bonds molecules A, B and C have covalent bonds
38. Napthalene sublimes because it has weak imf's
39. Non polar molecules don't dissolve in polar water
40. 128 is twice the mass of C5H4 so the molecular formula is C10H8
41. Ca: 42. :Cl: the Cl should have 7 dots 43. Ca+2 2[:Cl:] -1 each Cl gets 8 dots
44. covalent
45. low melting point is caused by weak attractive forces between particles
46. non conductors do not contain mobile charges
47. sulfur (they both will have 18 electrons)
48. carbon dioxide has symmetrical charge distribution which makes it non polar
49. the C-O bond has a greater difference in electronegativity than a F-F bond